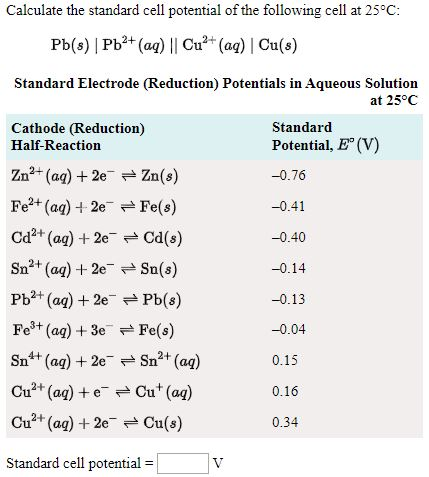
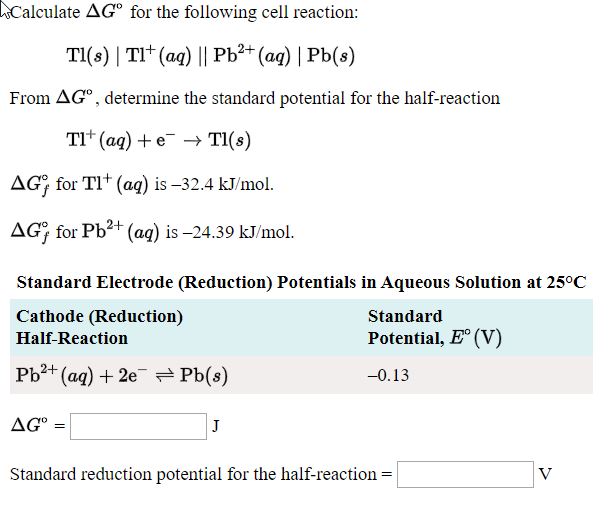
![SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of](https://cdn.numerade.com/ask_images/bd798f86fb6f4631925af95715b183c5.jpg)
SOLVED: Calculate the EMF of cells A through F at standard states (E"cel) and non-standard states (Eccll) using Nernst equation. Fill in Table-] with your answers b The Standard Electrode Potential of

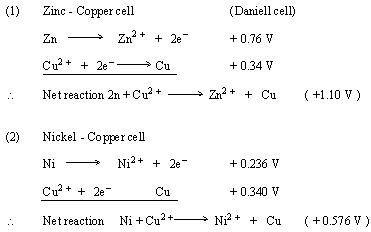
Ni | Ni^2 + || Cu^2 + | Cu The standard EMF of the above cell is 0.59 V. The standard electrode potential (reduction potential) of the copper electrode is 0.34 V.
How would you determine the standard electrode potential of Mg^2+/Mg? - Sarthaks eConnect | Largest Online Education Community
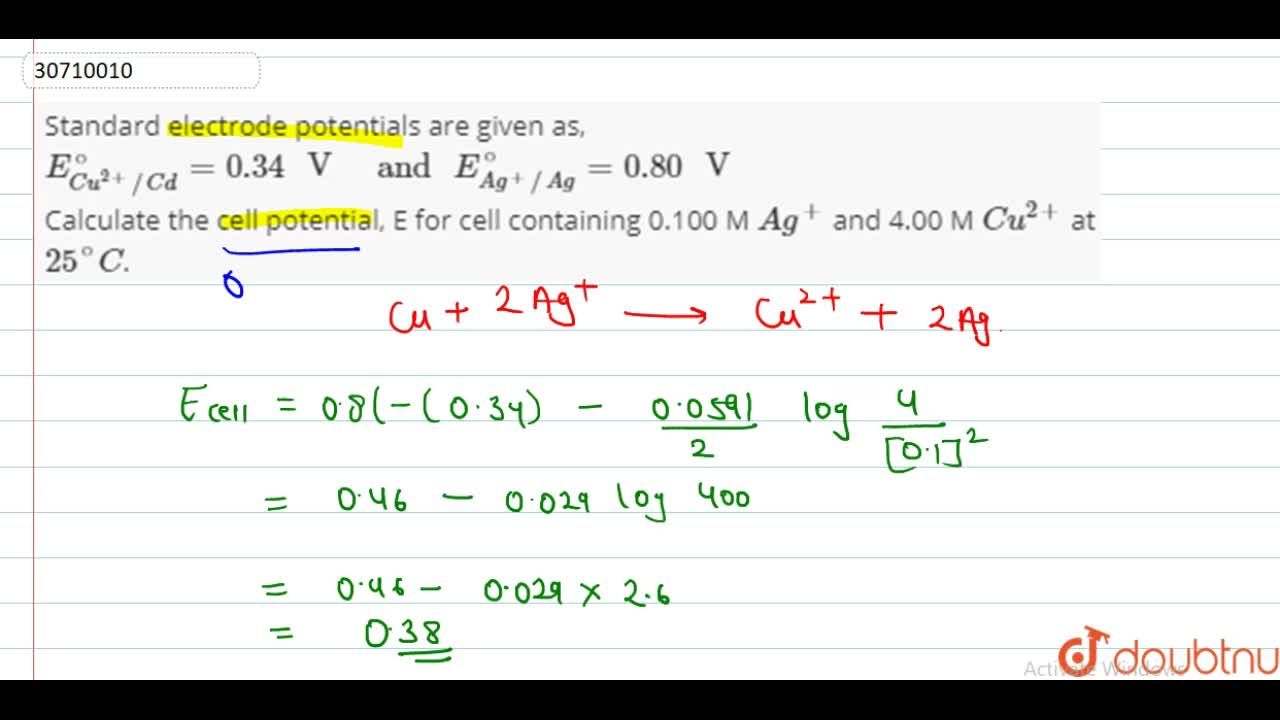
Standard electrode potentials are given as, E(Cu^(2+)//Cd)^(@)=0.34" V " " and " E(Ag^(+)//Ag)^(@)=0.80" V " Calculate the cell potential, E for cell containing 0.100 M Ag^(+) and 4.00 M Cu^(2+) at 25^(@)C.

Standard Reference Electrode Standard Hydrogen Electrode (SHE) SHE: Assigned V Can be anode or cathode Pt does not take part in reaction Difficult. - ppt download

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential potential of the cell, Ni//N^(2+)(0.01 M)//Cu is 0.59" V ". "Given" E(Cu^(2+)//Cu)^(@)=+0.34 " V "

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential ... - YouTube

Calculate the standard cell potentials of galvanic cell in which the following reactions take place:(i) 2Cr(s) + 3Cd^2 + (aq) → 2Cr^3 + (aq) + 3Cd (ii) Fe^2 + (aq) + Ag^+ (